Aer Therapeutics, a biopharmaceutical spin-out company and a collaboration between University College Dublin (UCD) and the University of California, San Francisco (UCSF), announced on Friday that it has secured $36M (approximately €32.7M) in a Series A round of funding.
The capital was received from a syndicate of premier life science industry investors, including Canaan, OrbiMed, and Hatteras Venture Partners.
Fund utilisation
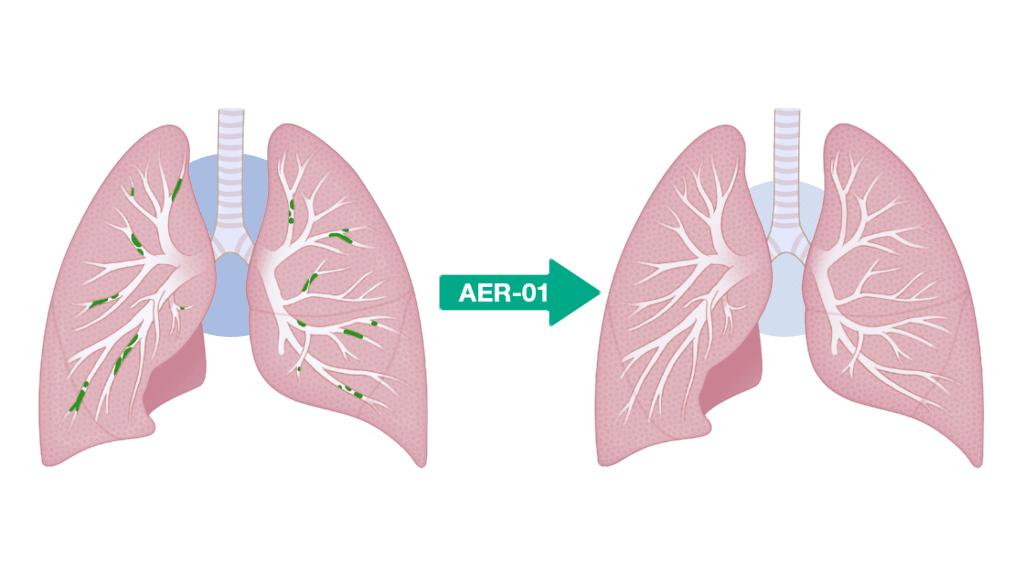
Aer Therapeutics says it will use the proceeds to further develop AER-01, the company’s inhaled small molecule mucolytic drug designed to liquefy mucus plugs in patients with chronic obstructive pulmonary disease (COPD).
The company plans to conduct a first-in-human Phase 1 clinical trial of AER-01 in mid-2023.
Jim Shaffer, President and CEO of Aer Therapeutics, says, “We are excited to introduce Aer Therapeutics as a company dedicated to delivering a therapeutic solution to patients with COPD who have severe airway obstruction caused by mucus plugs.”
“Aer will continue to leverage this expertise in the development of AER-01 and other therapeutic candidates for the treatment of muco-obstructive lung diseases,” adds Shaffer.
As part of the Series A financing, the company has expanded its board of directors to include the following new appointees:
- Tim Shannon, M.D., general partner at Canaan
- Rishi Gupta, J.D., partner at OrbiMed
- Christy Shaffer, Ph.D., general partner at Hatteras Venture Partners Thomas Mathers, CEO at Allievex
Treatment for mucus-associated lung diseases
Aer Therapeutics was co-founded by Professor John Fahy, UCSF, originally from Dublin, and Professor Stefan Oscarson, UCD School of Chemistry.
Aer Therapeutics is a clinical stage biopharmaceutical company that aims to develop novel treatments for mucus-associated lung diseases.
Professor Fahy’s laboratory at UCSF developed AER-01 with Professor Oscarson’s glycochemistry laboratory at UCD in collaboration with Professor Anne Marie Healy’s pharmaceutical technology laboratory at Trinity College Dublin.
The combined expertise of these laboratories in mucus biology, glycochemistry, inhaled drug formulation, drug delivery, and lung imaging, supported by a development grant from the National Institutes of Health, underlies the novel AER-01 technology.
Phase 1 clinical trials of its lead drug candidate AER-01 will start in 2023 with the potential to expand development into multiple other indications such as asthma, bronchiectasis, and cystic fibrosis.
What is AER-01?
AER-01 is a thiol-modified carbohydrate (“thiol-saccharide”) that cleaves mucin disulfide bridges to liquefy (“lyse”) mucus plugs.
Carbohydrate scaffolds are natural and non-toxic – their polar nature and high aqueous solubility allow them to easily penetrate mucus plugs.
AER-01 is a potent and fast-acting mucolytic well-suited for both nebulizer and dry powder delivery.
John Fahy, M.D., M.Sc., Professor of Medicine at UCSF and founder of Aer Therapeutics, says, “Studies using CT lung scans confirm that mucus plugs are highly prevalent in COPD patients, and those with a high mucus plug burden have lower lung function, increased frequency of exacerbations, diminished quality of life, and increased risk of all-cause mortality.”
“These findings provide a basis to specifically treat and remove mucus plugs as a strategy to improve lung health for COPD patients,” adds Fahy.
The compound is supported by a broad preclinical data package generated with the support of a five-year translational programme project grant (tPPG) to Drs. Fahy, Oscarson, and Healy from the National Institutes of Health (National Heart Lung and Blood Institute).
Professor Stefan Oscarson, UCD School of Chemistry and co-founder of Aer Therapeutics, says, “It is exciting times for me when after decades of academic research involving drug and vaccine development with colleagues at UCD, UCSF, and TCD, to see a lead drug candidate moving into human clinical trials. The AER-01 mucolytic drug has the potential to meet a wide range of clinical needs and to make a difference to many lives.”










01
From telecom veteran to Dutch Startup Visa success: The Jignesh Dave story